Introduction
--- ## 1. Direct Lithium Extraction (DLE) & Water Efficiency
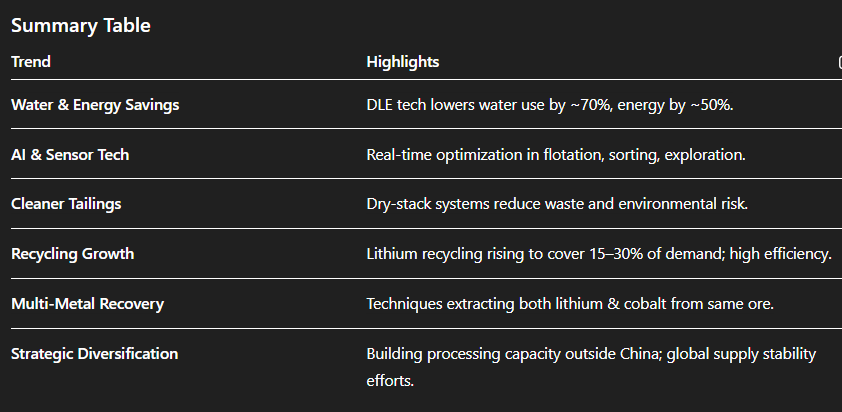
* **Direct lithium extraction (DLE)** technologies are becoming mainstream, growing at \~40% yearly since 2022. They reduce water use by \~70% and energy by \~50% compared to traditional evaporation methods, achieving higher lithium recovery rates (80–90% vs 40–50%) while lowering costs to \~\$3,500–\$4,500 per ton ([Discovery Alert][1], [IEA][2]).
* In places like South America, major lithium projects are adopting DLE with ion-exchange membranes, achieving \~93% water recycling and \~95% recovery, despite \~25% higher capital outlay ([P Market Research][3]).
--- ## 2. AI & Sensor-Driven Process Optimization
* Machine learning is optimizing reagent dosing in flotation circuits, boosting efficiency by up to \~30% while reducing environmental impact by \~20% ([Farmonaut®][4]).
* AI-enabled sorting with technologies like XRF and hyperspectral sensors enables selective recovery of lithium and cobalt, with efficiency gains of \~30% and environmental impact cut by \~35% ([Farmonaut®][4]).
* AI also enhances geological exploration—reducing drilling costs by up to 60% and quadrupling success rates, thus improving upstream discovery efficiency ([IEA][2]).
--- ## 3. Sustainable Processing & Reduced Environmental Footprint
* Regulations (e.g., EU’s Battery Regulation, Canada’s Tailings Act) now require 40–60% less freshwater use and up to 85% lower toxic tailings discharge, compelling investments toward cleaner beneficiation systems ([P Market Research][3]).
* Tailings management now accounts for \~22–30% of new project budgets—up from 8–12% pre-2020—as companies adopt filter-press (dry-stack) technologies that shrink waste volumes by \~60% and minimize acid drainage risks ([P Market Research][3]).
--- ## 4. Recycling, Urban Mining & Battery Material Reuse
* Lithium recycling is scaling: it's forecasted to cover 15–30% of global demand by 2035 (up from \~5% today). Advanced hydrometallurgical methods now achieve \~95% lithium recovery from battery black mass, outperforming older pyrometallurgical techniques (60–70%) ([Discovery Alert][1], [Metal.com][5]).
* Environmentally, recycled lithium uses \~70% less water and generates \~5.5 t CO₂ per ton versus \~15 t CO₂ for primary lithium ([Discovery Alert][6]).
--- ## 5. Innovations in Cobalt & Multi-Metal Recovery
* In Australia, emerging technologies enable **dual extraction** of lithium and cobalt from spodumene via AI-driven systems (e.g., Minvera Smart Dig), enhancing both yield and environmental efficiency while sharing infrastructure and water usage ([Farmonaut®][7]).
* In the DRC, many cobalt refiners now employ sensor-based sorting (e.g., by Tomra), reducing energy use by \~35% and delivering ESG-traceable data to comply with EU sustainability mandates ([P Market Research][3]).
--- ## 6. Geopolitics & Strategic Supply Chain Shifts
* China currently dominates lithium processing. In response, Western nations are investing heavily in domestic and allied processing hubs, diversifying supply chains ([Metal.com][5], [Reddit][8], [PwC][9]).
* The idea of a **Global Minerals Trust** (called for by the UN) aims to stabilize critical mineral supply (including lithium and cobalt), encourage sustainability, and support developing countries’ economies—but faces geopolitical hurdles ([The Wall Street Journal][10]).
--- ### Summary
Direct Lithium Extraction
Direct Lithium Extraction (DLE) is rapidly transforming how lithium is recovered, especially from brines. Unlike traditional evaporation ponds—which can take 12–18 months and consume massive volumes of water—DLE uses selective chemical, adsorption, or membrane processes to pull lithium directly from the brine, returning most of the water back underground.
--- ### **1. How It Works**
* **Feed Source:** Primarily brines from salt flats, geothermal waters, or oilfield brines.
* **Selective Extraction:** Uses ion-exchange resins, solvent extraction, or electrochemical membranes to capture lithium ions.
* **Water Recycling:** After lithium is removed, the remaining brine is reinjected into the aquifer, minimizing evaporation losses.
* **Concentration & Refining:** The captured lithium is purified into lithium carbonate or lithium hydroxide.
--- ### **2. Water Efficiency Gains**
* **Water Use Reduction:** DLE can cut freshwater consumption by \~70% compared to pond evaporation.
* **Closed-Loop Systems:** Many DLE setups recycle >90% of process water.
* **Aquifer Preservation:** Because the brine is returned, aquifer depletion is significantly reduced—crucial in arid regions like the Atacama Desert.
--- ### **3. Recovery & Yield Advantages**
* **Higher Recovery Rates:** Traditional evaporation yields \~40–50% lithium recovery; DLE can achieve 80–90%+.
* **Faster Processing:** Lithium extraction in hours or days instead of months.
* **Reduced Land Footprint:** Eliminates the need for massive evaporation ponds, freeing land and reducing environmental disturbance.
--- ### **4. Environmental & ESG Benefits**
* **Lower Carbon Footprint:** Less infrastructure and shorter process time reduce CO₂ emissions by \~20–40%.
* **Reduced Ecological Impact:** Less surface disturbance and brine handling minimizes harm to sensitive ecosystems.
* **Regulatory Compliance:** Meets stricter water-use and environmental laws, such as the EU Battery Regulation and Chile’s brine extraction limits.
--- ### **5. Challenges to Overcome**
* **Higher Capital Costs:** Early adoption costs are \~20–25% higher than evaporation ponds.
* **Complex Chemistry:** Brines with high magnesium, boron, or other impurities can reduce extraction efficiency.
* **Scaling Risks:** Commercial-scale operations are still in early phases—pilot plants are common, but full industrial adoption is emerging now.
---

AI and sensor driven Process Optimization
--- ### **1. Core Technologies**
* **Machine Learning Models:** Predict optimal reagent dosages, grinding settings, or separation parameters based on historical and real-time data.
* **Advanced Sensors:**
* **XRF (X-ray Fluorescence)** – Detects element composition for sorting ore.
* **Hyperspectral Imaging** – Identifies mineralogy on conveyors or stockpiles.
* **LIBS (Laser-Induced Breakdown Spectroscopy)** – Measures lithium content instantly.
* **Vibration & Acoustic Sensors** – Monitor grinding mill and pump health.
* **Process Control Systems:** Automated feedback loops adjust variables in flotation, leaching, or DLE circuits without operator delay.
--- ### **2. Key Benefits**
* **Higher Recovery & Throughput:**
* AI-assisted flotation can boost lithium recovery by **up to 30%** while lowering reagent use.
* Sensor-based sorting can reject low-grade material early, improving feed grade for downstream processes.
* **Lower Costs & Environmental Impact:**
* Optimized reagent dosing can cut chemical usage by **10–25%**.
* Sorting systems reduce energy per ton processed by **15–35%** by avoiding unnecessary grinding of waste.
* **Predictive Maintenance:**
* AI predicts equipment wear, preventing unplanned downtime and extending asset life.
--- ### **3. Lithium & Cobalt-Specific Applications**
* **Lithium Processing:**
* AI controls temperature, flow, and chemical concentration in **Direct Lithium Extraction (DLE)** systems to maintain high recovery efficiency.
* Sensor systems detect lithium-bearing minerals in spodumene ore before crushing, improving plant feed control.
* **Cobalt Processing:**
* Real-time ore sorting to separate cobalt-rich ore from barren gangue in Democratic Republic of Congo (DRC) mines.
* AI monitors leach chemistry in hydrometallurgical refining, keeping cobalt recovery >95% while minimizing acid consumption.
--- ### **4. Example in Action**
* **Tomra Sorting Solutions** installed AI-driven XRF sorters at a cobalt mine in Africa:
* Reduced energy consumption by \~35%.
* Increased cobalt grade in plant feed by \~20%.
* Provided full traceability data for ESG compliance in the EU battery supply chain.
--- ### **5. Challenges**
* **Data Quality & Volume:** AI needs high-quality training data, which is scarce in new projects.
* **Integration Costs:** Retrofitting older plants with sensor infrastructure can be expensive.
* **Workforce Skills:** Operators need training to interpret and trust AI-driven recommendations.
Sustainable Processing
--- ### **1. Water Stewardship**
* **Closed-Loop Water Systems:** Plants recycle 80–95% of process water, crucial in water-stressed regions like the Atacama Desert (lithium brines) or DRC mining hubs (cobalt).
* **Dry Processing Where Possible:** For certain lithium hard rock ores, dry crushing and sensor-based sorting reduce water demand before wet processing.
* **DLE Water Efficiency:** Direct Lithium Extraction reinjects processed brine underground, cutting freshwater use by \~70% compared to evaporation ponds.
--- ### **2. Tailings & Waste Reduction**
* **Dry-Stack Tailings:** Filter-press technology produces stackable, low-moisture tailings, reducing acid mine drainage risk and footprint by up to 60%.
* **Valorisation of By-products:** Recovery of potassium, magnesium, and boron from brines or tailings turns waste streams into revenue streams.
* **Reduced Reagent Toxicity:** Shift from hazardous solvents to biodegradable or low-impact reagents in flotation and leaching.
--- ### **3. Carbon Footprint Management**
* **Renewable Energy Integration:** Solar-powered DLE plants in Chile and geothermal-powered lithium extraction in Nevada cut Scope 2 emissions dramatically.
* **Electrified Mining Fleets:** Battery-electric haul trucks and loaders reduce diesel consumption in cobalt and lithium mines.
* **Process Efficiency Gains:** AI-optimized milling and flotation lower energy consumption per ton by 15–25%.
--- ### **4. Regulatory & ESG Drivers**
* **EU Battery Regulation:** Mandates carbon footprint declarations and recycling content for lithium, cobalt, nickel by 2027.
* **Global Tailings Standard:** Requires transparent reporting, risk management, and community safety plans for tailings storage facilities.
* **Investor Pressure:** ESG-focused funds increasingly screen for low-carbon, low-impact processing technologies before investing.
--- ### **5. Examples in Action**
* **Lithium Americas – Thacker Pass (USA):** Plans to use geothermal power and closed-loop water circuits to achieve one of the lowest carbon footprints per tonne of lithium carbonate.
* **CMOC – Tenke Fungurume (DRC):** Implemented dry-stack tailings and cobalt recovery upgrades, reducing process waste by \~30% and meeting EU supply chain traceability standards.
--- This shift means the next generation of lithium and cobalt plants will **look more like chemical refineries with recycling loops** than traditional mines—prioritizing *resource efficiency, circularity, and transparency*.
Recycling
With demand for lithium and cobalt projected to **triple or quadruple by 2035**, mining alone cannot meet future needs without severe environmental and social strain. Recycling and “urban mining” — recovering metals from end-of-life products, especially EV batteries — are becoming essential to secure supply, cut costs, and lower carbon footprints.
--- ### **1. Why It Matters**
* **Resource Security:** Recycling could meet **15–30% of global lithium and cobalt demand by 2035** (vs \~5% today).
* **Carbon Savings:** Producing lithium from recycled batteries emits \~5.5 t CO₂ per tonne vs \~15 t CO₂ from virgin mining.
* **Water Savings:** Hydrometallurgical recycling consumes \~70% less water than primary mineral extraction.
* **Circular Economy Compliance:** Regulations like the EU Battery Regulation require minimum recycled content (16% cobalt, 6% lithium by 2031).
--- ### **2. Key Recycling Methods**
**a) Hydrometallurgical Processing**
* Uses acids and solvents to leach metals from shredded battery “black mass.” * Can recover **>95% of lithium, cobalt, and nickel**.
* Lower energy use compared to smelting and enables selective recovery.
* Example: **Li-Cycle** (Canada) uses a water-based “Spoke and Hub” system with near-zero solid waste output. **b) Pyrometallurgical Processing**
* High-temperature smelting extracts cobalt, nickel, and copper; lithium often ends up in slag unless followed by hydromet steps.
* Robust for mixed chemistries but energy intensive.
* Example: Umicore’s Hoboken plant processes 7,000+ tonnes of battery material annually. **c) Direct Recycling (Cathode-to-Cathode)**
* Maintains original cathode crystal structure, reducing reprocessing needs.
* Still emerging but could cut both cost and energy by avoiding full chemical breakdown.
--- ### **3. Urban Mining Beyond Batteries**
* **Electronics:** Old laptops, phones, and e-bikes are significant cobalt and lithium sources.
* **Industrial Scrap:** Off-spec cathode material and process residues from gigafactories are already being fed back into the supply loop.
* **EV & Stationary Storage Packs:** Second-life applications (e.g., grid storage) extend useful battery life before recycling.
--- ### **4. Challenges to Scale**
* **Collection & Logistics:** Many end-of-life batteries are not tracked or returned for recycling.
* **Safety Risks:** Damaged lithium-ion cells can catch fire during transport or shredding.
* **Chemistry Variability:** Different cathode types (LFP, NMC, NCA) complicate process optimization.
* **Economics:** Recycling profitability is tied to cobalt prices; lithium recovery alone is often marginal unless incentivized.
--- ### **5. Example Projects**
* **Redwood Materials (USA):** Targets 100 GWh/year of battery recycling capacity by 2025, sourcing feed from Tesla, Panasonic, and Ford.
* **CATL (China):** Integrated battery manufacturing and recycling with >90% lithium recovery and >99% cobalt recovery.
* **Fortum (Finland):** Uses low-CO₂ hydromet processes to achieve >80% total material recovery.
Innovation in Cobalt and Multi Mineral Recovery
Cobalt rarely occurs in isolation — it’s usually a by-product of copper or nickel mining, and increasingly, lithium operations are also considering cobalt co-recovery from certain pegmatites and brines. Recent innovations aim to **maximize the recovery of all valuable metals from the same ore stream**, lowering costs and environmental impact while improving supply security.
--- ### **1. Integrated Processing Flowsheets**
* **Co-Processing Copper & Cobalt:**
* In the DRC, new hydrometallurgical plants process mixed copper-cobalt concentrates in the same leach circuit.
* Refining sequences are adjusted to extract copper first, then cobalt from the raffinate, often using **solvent extraction + electrowinning (SX-EW)**.
* Benefits: shared infrastructure, lower reagent consumption, and reduced waste volumes.
* **Lithium-Cobalt Dual Recovery:**
* Some spodumene pegmatites contain trace cobalt minerals; innovative flotation and selective leaching stages allow both lithium and cobalt recovery.
* This “multi-metal DLE” approach is being tested in Australia, pairing adsorption media for lithium with chelating resins for cobalt in one circuit.
--- ### **2. Sensor-Based Ore Sorting for Feed Upgrading**
* **XRF & Hyperspectral Sorting:**
* Detect cobalt minerals (carrollite, heterogenite) in ore before grinding, upgrading feed grade by **15–25%**.
* Reduces energy costs by avoiding processing of barren rock. * Example: TOMRA sorters in African cobalt mines have cut milling energy use by \~35% while increasing cobalt yield.
--- ### **3. Advanced Leaching & Selective Extraction**
* **Chloride Leaching:** More effective for refractory cobalt ores and allows for simultaneous recovery of other metals like manganese and nickel.
* **Ionic Liquid & Bio-Leaching:**
* Bacteria like *Acidithiobacillus ferrooxidans* enhance cobalt liberation from low-grade ores and tailings. * Ionic liquids selectively dissolve cobalt, leaving other impurities behind, reducing downstream purification steps.
--- ### **4. By-Product Recovery from Tailings & Waste**
* **Tailings Re-Processing:**
* Many older copper tailings in the DRC and Zambia still contain 0.1–0.3% cobalt.
* Modern leach and SX-EW circuits can economically recover these metals while remediating legacy waste sites.
* **Battery Recycling as a Cobalt Source:**
* Cobalt recovery rates from battery black mass exceed 95% with hydrometallurgical processing, now supplementing mined supply.
--- ### **5. ESG & Traceability Innovations**
* **Blockchain Tracking:** Companies like CMOC and Glencore are implementing blockchain to track cobalt from mine to battery cell, ensuring compliance with EU and US ethical sourcing requirements.
* **Low-Impact Energy Sources:** Hydromet plants in Morocco and Finland are integrating wind or hydroelectric power to reduce Scope 2 emissions by over 40%.
--- ### **6. Example in Action**
* **Tenke Fungurume Mining (DRC):**
* Upgraded leach plant with SX-EW cobalt recovery modules.
* Introduced ore sorting ahead of milling.
* Increased cobalt recovery by \~20% while reducing acid consumption by \~15%.
Geopolitics and Strategic Supply Chain Shifts
As electric vehicles, renewable energy storage, and defense industries accelerate demand, governments and companies are reshaping mining, processing, and trade flows to secure access — while reducing dependence on a handful of dominant suppliers.
--- ### **1. Current Supply Concentration**
* **Lithium Processing Dominance:**
* China processes **\~60–70%** of the world’s lithium chemicals despite mining only \~15% of raw lithium ore/brine.
* Australia, Chile, and Argentina mine most of the raw lithium but rely heavily on Chinese refineries.
* **Cobalt Supply Dependence:**
* **\~70%** of mined cobalt comes from the Democratic Republic of Congo (DRC).
* China refines over **75%** of global cobalt chemicals used in batteries.
--- ### **2. Strategic Responses from Other Regions**
* **United States:** * Inflation Reduction Act (IRA) offers subsidies for battery materials sourced from “allied nations.”
* Investments in domestic lithium refining (e.g., Albemarle, Lithium Americas) and cobalt recycling (e.g., Redwood Materials).
* **European Union:**
* EU Critical Raw Materials Act sets targets to extract 10%, process 40%, and recycle 15% of strategic minerals domestically by 2030.
* New trade deals with Argentina, Chile, and Namibia for lithium and cobalt supply.
* **Japan & South Korea:**
* Long-term offtake agreements and joint ventures in Africa, South America, and Australia.
--- ### **3. Supply Chain Diversification & Friend-Shoring**
* **Multi-Hub Processing:** New refineries in Australia, Finland, Canada, and Morocco aim to bypass Chinese processing.
* **Integrated Mining-to-Refining Projects:** Some lithium and cobalt miners now build their own downstream plants to keep more value onshore.
* **Joint Ventures:** Automakers like Tesla, GM, and VW are securing direct offtake agreements and investing in mining/recycling companies.
--- ### **4. ESG & Human Rights Pressures**
* Artisanal cobalt mining in the DRC has raised serious human rights and child labor concerns.
* Western OEMs increasingly require **traceability systems** (blockchain, IoT tagging) to prove ethical sourcing.
* Regulations such as the **US Uyghur Forced Labor Prevention Act** are influencing battery supply chain audits.
--- ### **5. Emerging Geopolitical Risks**
* **Resource Nationalism:** Countries like Chile and Indonesia are tightening control over lithium and nickel resources.
* **Export Controls:** China recently introduced export permit requirements for certain graphite and rare earth processing tech, raising concerns it could do the same for lithium or cobalt.
* **Global Minerals Trust Idea:** Proposed by the UN to stabilize supply and promote sustainable mining in developing nations — but implementation faces political resistance.
--- ### **6. Example in Action**
* **Morocco’s Cobalt Strategy:** Leveraging Managem’s Bou Azzer mine (the only primary cobalt mine outside the DRC) and building processing plants to supply Europe, reducing reliance on Chinese refiners.
--- This geopolitical reshaping means that **future lithium and cobalt supply chains will be judged not just by price and capacity, but also by location, traceability, and ESG compliance**.
How the Lithium and cobalt supply Chain shift works
--- ### **1. Raw Material Extraction**
* **Lithium:** * **Hard rock (spodumene)** mining in Australia, Canada.
* **Brine extraction** in Chile, Argentina, Bolivia (Lithium Triangle).
* **Cobalt:** * Mostly as a **by-product of copper mining** in the DRC and nickel mining in Indonesia, Australia, and Russia.
* Governments in resource-rich countries set **royalties, export quotas, or state ownership stakes** to secure national benefits.
--- ### **2. Concentration & Intermediate Products**
* Ore or brine is processed into concentrates (e.g., spodumene concentrate for lithium, cobalt hydroxide for cobalt).
* Some countries ship concentrates abroad for further refining, especially to **China**, which dominates the next stage.
--- ### **3. Chemical Processing & Refining**
* Lithium concentrate → **lithium carbonate** or **lithium hydroxide** (battery-grade).
* Cobalt hydroxide → **cobalt sulfate** for cathode production.
* New processing plants in **Australia, Morocco, Canada, and Finland** aim to **short-circuit China’s dominance**.
* Technology choices like **Direct Lithium Extraction (DLE)** and **hydrometallurgical cobalt refining** are adopted to reduce water, energy, and waste.
--- ### **4. Cathode & Battery Manufacturing**
* Battery-grade chemicals go to **cathode active material (CAM)** producers, then cell manufacturers.
* Regions like the **EU, US, and Japan** are building domestic gigafactories but still rely on imported refined chemicals unless local refining ramps up.
--- ### **5. Supply Chain Diversification (Friend-Shoring)**
* Countries form trade alliances to **source from politically aligned nations**.
* Example: The US sources more lithium from Australia and Chile, and cobalt from Morocco and Canada, bypassing Chinese refiners.
* Automakers sign **long-term offtake agreements** directly with miners and refiners to secure supply.
--- ### **6. Recycling & Urban Mining Loop** * End-of-life batteries are collected and processed through **hydrometallurgical or pyrometallurgical recycling**.
* Recovered lithium and cobalt re-enter the battery supply chain, reducing dependence on fresh mining.
--- ### **7. Traceability & ESG Compliance**
* Blockchain and IoT tagging systems track each batch of metal from mine to battery.
* Complies with laws like the **EU Battery Regulation** and **US sourcing rules** for EV tax credits.
* Buyers increasingly require **proof of ethical sourcing** to avoid reputational risk.
Raw material extraction for lithium and cobalt
--- ## **1. Lithium Extraction Methods**
### **a) Hard Rock Mining**
* **Source:** Spodumene pegmatites in Australia, Canada, Zimbabwe, and Portugal.
* **Process:**
1. Open-pit mining of ore.
2. Crushing and dense media separation (DMS) or flotation to produce spodumene concentrate (\~6% Li₂O).
3. Concentrate shipped to refineries for chemical conversion.
* **Advantages:** High-grade ore, stable supply.
* **Challenges:** High energy use, requires chemical conversion to battery-grade compounds.
### **b) Brine Extraction**
* **Source:** Salt flats (salars) in Chile, Argentina, Bolivia; also geothermal brines in the US and Europe.
* **Traditional Method:** Pump brine to evaporation ponds for 12–18 months, concentrate lithium salts, then process to lithium carbonate.
* **Modern Method (DLE):** Extract lithium directly from brine using ion-exchange, adsorption, or membrane tech, then reinject brine underground.
* **Advantages:** Lower mining footprint, scalable with water recycling.
* **Challenges:** Sensitive to water rights and ecosystem impacts.
--- ## **2. Cobalt Extraction Methods**
### **a) By-Product of Copper Mining**
* **Source:** Copper–cobalt deposits in the Democratic Republic of Congo (DRC), Zambia.
* **Process:**
1. Open-pit or underground mining.
2. Crushing, milling, and flotation or leaching to separate copper.
3. Cobalt recovered from the copper circuit’s raffinate via solvent extraction or precipitation.
* **Advantages:** Shared infrastructure with copper, high cobalt yield.
* **Challenges:** ESG concerns — artisanal mining, child labor, political instability.
### **b) By-Product of Nickel Mining**
* **Source:** Nickel–cobalt laterites in Indonesia, Australia, Philippines, and Russia.
* **Process:**
1. Mining laterite ore (often open-pit).
2. High-pressure acid leach (HPAL) to dissolve nickel and cobalt.
3. Separation and refining to cobalt sulfate or metal.
* **Advantages:** Large, long-life deposits.
* **Challenges:** HPAL is capital-intensive and energy-heavy.
--- ## **3. Environmental & Social Considerations**
* **Water Use:** Brine extraction can strain aquifers; hard rock mining consumes significant process water.
* **Carbon Footprint:** HPAL and spodumene roasting have high CO₂ emissions unless renewable energy is used.
* **Community Impact:** Land use conflicts, artisanal mining safety, and benefit sharing are increasingly scrutinized.
Concentration and intermediate Products
--- ## **1. Lithium Concentration**
### **a) From Hard Rock (Spodumene)**
* **Crushing & Grinding:** Ore reduced to liberation size.
* **Dense Media Separation (DMS):** Removes low-density waste rock; yields a spodumene-rich fraction.
* **Flotation:** Further upgrades lithium minerals, producing **spodumene concentrate** (\~5.5–6.5% Li₂O).
* **Intermediate Product:**
* **Spodumene Concentrate** — shipped to conversion plants for calcination, acid roasting, and conversion to lithium carbonate or hydroxide.
* **By-products:** Feldspar, quartz, tantalum concentrates.
### **b) From Brine**
* **Traditional Evaporation Route:** Concentrates lithium salts in ponds to >1% Li by removing water and precipitating unwanted salts (NaCl, KCl, MgCl₂).
* **Direct Lithium Extraction (DLE):** Uses selective sorbents, membranes, or solvents to capture lithium ions directly from brine, producing **lithium-rich eluate**.
* **Intermediate Product:**
* **Lithium Concentrate Solution** — sent for purification and crystallization into lithium carbonate/hydroxide.
--- ## **2. Cobalt Concentration**
### **a) Copper–Cobalt Ores (DRC, Zambia)**
* **Flotation:** Separates copper and cobalt sulfides from gangue.
* **Leaching:** Dissolves copper and cobalt into solution.
* **Solvent Extraction (SX):** First recovers copper; cobalt remains in the raffinate.
* **Intermediate Product:**
* **Cobalt Hydroxide** (20–40% Co) or **cobalt carbonate** — shipped to refineries for conversion to cobalt sulfate.
### **b) Nickel–Cobalt Laterites (Indonesia, Australia)**
* **HPAL (High-Pressure Acid Leach):** Leaches nickel and cobalt from laterite ore.
* **Solution Purification:** Removes iron, aluminum, magnesium.
* **Intermediate Product:**
* **Mixed Hydroxide Precipitate (MHP)** — \~35–45% Ni + Co combined.
* **Mixed Sulfide Precipitate (MSP)** — similar grades, different chemistry.
--- ## **3. Why Intermediates Matter**
* **Transport Efficiency:** Shipping concentrates or precipitates is far cheaper than shipping raw ore/brine due to lower mass and higher metal content.
* **Market Flexibility:** Intermediates can be sold to multiple refiners worldwide.
* **Quality Control:** Early removal of impurities lowers downstream refining costs and improves product consistency.
--- ## **4. Examples**
* **Pilbara Minerals (Australia):** Produces spodumene concentrate for shipment to China and local chemical plants.
* **CMOC Tenke Fungurume (DRC):** Produces cobalt hydroxide as a by-product of copper SX-EW.
* **PT Halmahera Persada Lygend (Indonesia):** Produces MHP from laterites for direct conversion to battery-grade cobalt sulfate.
Chemical Processing and refining
After concentration into high-grade intermediates, lithium and cobalt undergo **chemical transformation** to produce **battery-grade chemicals** (e.g., lithium carbonate/hydroxide and cobalt sulfate).
This stage is where metallurgical precision directly impacts EV battery performance.
--- ## **1. Lithium Refining**
### **a) From Spodumene Concentrate**
1. **Calcination (Conversion):**
* Spodumene α-phase → β-phase at \~1,050 °C to make lithium more reactive.
2. **Acid Roasting:**
* β-spodumene mixed with sulfuric acid and roasted (\~250 °C) to form lithium sulfate.
3. **Leaching:**
* Lithium sulfate dissolved in water; impurities filtered out.
4. **Purification:**
* Removal of magnesium, calcium, and iron via precipitation.
5. **Precipitation of Lithium Compounds:**
* **Lithium Carbonate:** Adding soda ash (Na₂CO₃).
* **Lithium Hydroxide:** Converting carbonate via caustic soda or directly from lithium sulfate using ion exchange/membrane processes.
6. **Drying & Packaging:** Battery-grade product (>99.5% purity).
--- ### **b) From Brine / DLE**
1. **Lithium Extraction:**
* Selective sorbents, membranes, or solvents produce a lithium-rich solution.
2. **Polishing:**
* Removal of boron, calcium, magnesium.
3. **Precipitation & Crystallization:**
* Lithium carbonate or hydroxide crystallized from purified solution.
4. **Drying & Bagging:** Ready for cathode production.
--- ## **2. Cobalt Refining**
### **a) From Cobalt Hydroxide (Copper–Cobalt ores)**
1. **Leaching:** * Cobalt hydroxide dissolved in sulfuric acid.
2. **Impurity Removal:** * Iron, manganese, and copper removed via pH-controlled precipitation.
3. **Solvent Extraction / Ion Exchange:** * Selectively separates cobalt from nickel and other metals.
4. **Crystallization:**
* Produces **cobalt sulfate heptahydrate** (CoSO₄·7H₂O), essential for NCM/NCA battery cathodes.
5. **Drying & Quality Control:** Battery-grade >20.5% Co content, low impurities.
--- ### **b) From MHP / MSP (Nickel–Cobalt Laterites)**
1. **Re-leaching:** * Dissolves MHP/MSP in acid.
2. **Sequential Extraction:**
* Removes nickel (if required) and isolates cobalt.
3. **Final Product:**
* Cobalt sulfate, cobalt oxide, or metallic cobalt powder, depending on demand.
--- ## **3. Key Trends in Chemical Refining**
* **Direct Lithium Hydroxide Production:** New spodumene flowsheets bypass carbonate step for cathode-ready hydroxide.
* **Closed-Loop Water Systems:** Minimizing water use and effluent discharge.
* **Modular Refineries:** Smaller, distributed refining units close to mines to reduce shipping volumes of low-value waste.
* **Green Chemistry Approaches:** Reagent recycling, renewable power, and CO₂-neutral operations.
* **Traceability & ESG Compliance:** Digital tracking from mine to battery under growing OEM and regulatory pressure.
Cathode and battery manufacturing
Once lithium and cobalt have been refined into **battery-grade chemicals** (like lithium carbonate, lithium hydroxide, and cobalt sulfate), they move into the **battery materials and cell manufacturing stage** — the bridge between mining and the EV/energy storage markets.
--- ## **1. Cathode Active Material (CAM) Production** This is where lithium, cobalt, nickel, manganese, and other elements are chemically combined into crystalline compounds for use in cathodes.
### **a) Precursor Production**
1. **Co-precipitation:**
* Nickel, cobalt, and manganese sulfates (or other metal solutions) are mixed in precise ratios.
* A controlled pH environment (ammonia, NaOH) precipitates uniform **NCM/NCA hydroxide precursors**.
2. **Washing & Filtration:**
* Removes impurities and ensures particle size consistency.
3. **Drying:** * Produces free-flowing hydroxide powder.
--- ### **b) Lithiation**
1. **Mixing:**
* Hydroxide precursor blended with **lithium carbonate or lithium hydroxide**.
2. **Calcination:**
* High-temperature kiln (\~700–900 °C) fuses lithium into the crystal lattice, forming layered oxide cathode material (e.g., **LiNiₓCoᵧMn𝓏O₂**).
3. **Post-Processing:**
* Grinding, sieving, and coating (e.g., with Al₂O₃) to improve stability and lifespan.
--- ### **c) Types of Cathode Materials**
* **NCM** (Nickel Cobalt Manganese) – balance of energy density, cost, and stability.
* **NCA** (Nickel Cobalt Aluminum) – high energy density, popular in Tesla vehicles.
* **LFP** (Lithium Iron Phosphate) – cobalt-free, longer cycle life, lower cost.
* **LMO** (Lithium Manganese Oxide) – high safety but lower capacity.
* **High-Mn & Cobalt-Free Chemistries** – emerging to reduce cobalt dependency.
--- ## **2. Battery Cell Manufacturing**
### **a) Electrode Fabrication**
1. **Cathode Slurry:**
* CAM mixed with binder (PVDF) and conductive carbon in N-Methyl-2-pyrrolidone (NMP) solvent.
* Coated onto aluminum foil, then dried.
2. **Anode Slurry:**
* Graphite (natural or synthetic), binder, and conductive carbon in water-based solvent.
* Coated onto copper foil.
3. **Calendaring:**
* Compresses electrodes to required density and porosity.
--- ### **b) Cell Assembly**
* **Separator Placement:** Microporous polymer film between cathode and anode.
* **Electrolyte Filling:** Usually lithium salts in organic carbonate solvents (LiPF₆ in EC/DEC).
* **Sealing:** Cells sealed in cylindrical, pouch, or prismatic casings.
--- ### **c) Formation & Aging**
1. **Formation Cycling:**
* First charge–discharge cycles to form a stable SEI (Solid Electrolyte Interphase).
2. **Aging:**
* Cells stored under controlled temperature to detect early failures.
--- ## **3. Key Trends in Cathode & Battery Manufacturing**
* **Cobalt Reduction:** Shifting to high-Ni or cobalt-free chemistries for cost and supply chain security.
* **Solid-State Batteries:** Eliminating liquid electrolytes for higher safety and energy density.
* **Direct Recycling Integration:** Closing the loop with
“cathode-to-cathode” recovery processes.
* **Localized Gigafactories:** Manufacturing close to demand centers to cut logistics costs.
* **AI & Automation:** Quality control, defect detection, and process optimization in real time.
* **Water & Solvent Recovery:** Especially NMP solvent recycling to reduce environmental impact.
Supply Chain Diversification
Friend-shoring is the strategic realignment of critical mineral supply chains toward **geopolitically aligned, stable, and trusted partner nations** to reduce dependence on high-risk suppliers. In lithium and cobalt, where production and refining are heavily concentrated in a few countries, this shift is reshaping investment, trade, and processing strategies.
--- ## **1. Why Friend-Shoring Matters**
* **Geopolitical Risks:** Over 70% of cobalt is mined in the DRC, with more than 60% refined in China; over 60% of lithium refining is also in China.
* **Supply Chain Resilience:** Disruption risks from trade disputes, political instability, or export restrictions.
* **Strategic Control:** Governments and OEMs want secure access to battery raw materials to meet EV and energy storage targets.
* **ESG Compliance:** Sourcing from partners with higher environmental, labor, and governance standards.
--- ## **2. Friend-Shoring in Practice**
### **a) Upstream (Mining)**
* Investments shifting to **Australia, Canada, Brazil, Chile, Argentina**, and **Namibia** for lithium.
* New cobalt projects in **Australia, Canada, Morocco**, and **Indonesia** (nickel-cobalt intermediates).
* Partnerships with African nations that meet OECD mineral traceability standards.
### **b) Midstream (Refining & Processing)**
* **Lithium hydroxide plants** being built in the US, EU, and Australia to bypass reliance on Chinese refiners.
* **Cobalt sulfate refining** expanding in Finland, Canada, and South Korea. * Regional value-add incentives (e.g., US Inflation Reduction Act) encouraging domestic processing.
### **c) Downstream (Battery & Cathode Manufacturing)**
* Gigafactories strategically located in **North America, Europe, and Japan/Korea** to ensure cell supply security.
* Cathode material plants colocated with refineries for faster feedstock-to-product turnaround.
--- ## **3. Key Enablers of Friend-Shoring**
* **Government Policy:** Tax credits, grants, import tariffs, and domestic content rules.
* **Strategic Alliances:** OEMs (Tesla, VW, GM) partnering directly with miners and refiners.
* **Long-Term Offtake Agreements:** Locking in supply from trusted jurisdictions.
* **ESG Certification:** Adoption of IRMA, RMAP, and other audit frameworks for verifiable responsible sourcing.
--- ## **4. Challenges & Considerations**
* **Higher Costs:** Production in friend-shored regions may be more expensive than in legacy hubs.
* **Capacity Gaps:** New mines and refineries take years to develop; short-term reliance on existing supply remains.
* **Technological Bottlenecks:** Need to transfer refining expertise from dominant players to new regions.
* **Logistics:** Some “friendly” sources are still geographically far from major manufacturing centers.
--- ## **5. Trends to Watch**
* **Regional Lithium Processing Clusters:** Australia–Korea, Chile–EU, US–Canada corridors.
* **Battery Alliance Initiatives:** EU Battery Alliance, US–Australia Critical Minerals Partnership.
* **Integrated Recycling Hubs:** To reduce virgin material demand and complement friend-shored supply.
* **Multi-Metal Processing:** Plants designed to recover lithium, nickel, cobalt, and manganese from diverse ores.
Recycling & Urban Mining Loop
The **Recycling & Urban Mining Loop** is the circular supply chain model that recovers valuable metals from end-of-life (EoL) batteries, manufacturing scrap, and other consumer electronics. It closes the resource loop, reduces reliance on virgin mining, and supports sustainability goals for lithium and cobalt supply.
--- ## **1. Why It Matters**
* **Resource Security:** Reduces dependence on geologically and geopolitically constrained mining operations.
* **Environmental Benefits:** Minimizes waste, lowers greenhouse gas emissions, and reduces water and land use.
* **Economic Viability:** Metal recovery from used batteries can be cheaper and faster than extracting from raw ore, especially as technology advances.
* **Policy Drivers:** EU Battery Regulation, US Inflation Reduction Act, and similar laws mandating recycling quotas and traceability.
--- ## **2. The Loop Process**
### **a) Collection & Reverse Logistics**
* **Sources:** End-of-life EV batteries, stationary storage systems, portable electronics, and production scrap.
* **Systems:** Dealer take-back programs, dedicated battery collection centers, OEM–recycler partnerships.
* **Challenges:** Safe transport, tracking battery chemistry, and ensuring compliance with hazardous materials regulations.
--- ### **b) Battery Disassembly & Preprocessing**
* **Manual & Automated Disassembly:** Removal of modules and cells from casings.
* **Pre-treatment Methods:**
* **Mechanical shredding** to produce “black mass.”
* **Thermal treatment** to deactivate and remove flammable electrolytes.
* **Cryogenic processing** to prevent fires during shredding.
--- ### **c) Material Recovery**
* **Hydrometallurgy:** Leaching black mass with acids or bioleaching to extract Li, Co, Ni, Mn.
* **Pyrometallurgy:** High-temperature smelting to recover cobalt, nickel, and copper; often followed by hydromet.
* **Direct Recycling:** Retains and rejuvenates cathode material without breaking it into base metals, preserving structure and reducing processing energy.
--- ### **d) Refining & Reuse**
* Refining recovered metals to **battery-grade lithium carbonate/hydroxide, cobalt sulfate, and nickel sulfate**.
* Supply to cathode active material (CAM) producers for new battery manufacturing.
* Residual metals (Cu, Al, Fe) sold to other industries.
--- ## **3. The Closed-Loop Advantage**
* **Faster Turnaround:** Metals re-enter the battery supply chain within months, versus years for mining.
* **Predictable Feedstock:** Recycling volumes grow as EV adoption rises, creating a stable secondary source.
* **Synergy with Friend-Shoring:** Recycling plants can be colocated with gigafactories and refineries in friendly jurisdictions, reducing transport risks.
--- ## **4. Future Outlook**
* **AI Sorting Systems:** Identify and classify batteries by chemistry for optimized recovery.
* **Urban Mining of Consumer Devices:** Smartphones, laptops, and e-bikes as significant cobalt sources.
* **Modular Processing Plants:** Smaller, distributed recycling facilities to reduce logistics costs.
* **Policy-Driven Markets:** OEMs increasingly required to use a % of recycled content in new batteries.
Treatability and ESG Compliance
Traceability and ESG (Environmental, Social, and Governance) compliance are becoming non-negotiable for lithium and cobalt, given the rising global demand for batteries and the scrutiny on how these critical minerals are sourced and processed.
The focus is not only on **where** the materials come from, but also **how** they are extracted, processed, transported, and reused.
--- ## **1. Why Traceability Matters**
* **Risk Management:** Ensures materials are not linked to child labor, unsafe mining practices, or environmental harm.
* **Regulatory Compliance:** Meets requirements such as the EU Battery Regulation, US SEC disclosures, and OECD Due Diligence Guidance for Responsible Mineral Supply Chains.
* **Customer & Investor Expectations:** OEMs, EV buyers, and institutional investors demand proof of ethical sourcing.
* **Market Access:** Non-compliant suppliers risk being excluded from major markets.
--- ## **2. Tools & Technologies**
* **Blockchain & Digital Ledgers:** Immutable records of every step from mine to battery pack.
* **Digital Product Passports:** QR or RFID tags linking physical products to verified sourcing and ESG performance data.
* **IoT & Sensor Integration:** GPS-enabled tracking for shipments; automated environmental monitoring at mine sites.
* **AI & Data Analytics:** Real-time ESG risk scoring for suppliers and operations.
--- ## **3. ESG Compliance Pillars**
### **Environmental**
* **Carbon Footprint Accounting:** Tracking GHG emissions for each tonne of lithium carbonate equivalent (LCE) or cobalt sulfate produced.
* **Water Use & Impact Monitoring:** Especially critical for lithium brine operations in arid regions.
* **Waste & Tailings Management:** Ensuring safe storage and rehabilitation.
### **Social**
* **Labor Standards:** No child or forced labor; fair wages and working conditions.
* **Community Engagement:** Shared benefits from mining and processing activities.
* **Indigenous Rights:** Respecting land claims and cultural heritage. ### **Governance**
* **Anti-Corruption Measures:** Transparent contracting and payments.
* **Third-Party Audits:** Verification of ESG reports by independent bodies.
* **Chain-of-Custody Certification:** E.g., RMI (Responsible Minerals Initiative), IRMA (Initiative for Responsible Mining Assurance).
--- ## **4. Integration into the Battery Supply Chain**
* **Mine to Market Tracking:** Each batch of lithium or cobalt is tagged with origin data, processing history, and ESG metrics.
* **Friend-Shoring Synergy:** Traceability supports alliances between jurisdictions with aligned environmental and labor standards.
* **Recycling Inclusion:** Closed-loop systems include recycled material tracking to prove origin and content percentage.
--- ## **5. Future Trends**
* Mandatory **digital product passports** in the EU by 2027 for all batteries.
* AI-driven ESG auditing that cross-checks satellite imagery, shipping records, and on-site data.
* Blockchain consortiums linking miners, refiners, OEMs, and recyclers.
* Investors tying financing rates to verified ESG performance.
Integrated Process Flowsheet
--- ## **Integrated Processing Flowsheets**
### **1. Co-Processing Copper & Cobalt**
**Context:** In the Democratic Republic of Congo (DRC), abundant copper-cobalt ore bodies enable the design of hydrometallurgical plants capable of processing both metals in a single flowsheet.
**Process Approach:**
* **Mixed Concentrates Feed:** Ore containing both copper and cobalt is crushed, milled, and concentrated through flotation.
* **Shared Leach Circuit:** The concentrate enters an acid leach stage where both copper and cobalt dissolve into solution.
* **Sequential Metal Recovery:**
1. **Copper First:** Solvent extraction (SX) removes copper, which is then recovered via electrowinning (EW).
2. **Cobalt Second:** The raffinate, now copper-free, undergoes cobalt SX-EW or precipitation to yield cobalt products.
**Advantages:**
* **Shared Infrastructure:** One plant processes two commodities, reducing CAPEX.
* **Lower Reagent Consumption:** Common leach chemistry minimizes chemical use.
* **Reduced Waste Volumes:** Tailings and residue streams are consolidated, lowering environmental footprint.
--- ### **2. Lithium-Cobalt Dual Recovery**
**Context:** Some spodumene pegmatite deposits contain trace cobalt minerals alongside lithium-bearing phases.
**Process Approach:**
* **Ore Preparation:** Crushing, milling, and classification to liberate spodumene and associated cobalt minerals.
* **Flotation & Separation:**
* Spodumene is concentrated using standard froth flotation.
* Cobalt minerals are selectively floated or recovered from flotation tailings.
* **Innovative Multi-Metal Leaching:**
* **Lithium Extraction:** Direct Lithium Extraction (DLE) media or roasting + leaching to recover lithium.
* **Cobalt Recovery:** Chelating ion-exchange resins selectively capture cobalt from leach solutions.
**Pilot Developments:**
* Trials in Australia are integrating **adsorption media for lithium** with **chelating resins for cobalt** in a single continuous circuit.
* Potential for **multi-metal DLE plants** that maximize revenue per tonne of ore and minimize separate processing streams.
Advanced Leaching and Selective Extraction
--- ## **Advanced Leaching & Selective Extraction**
**Overview:** Modern lithium and cobalt projects are moving beyond conventional acid leaching to more **selective, efficient, and environmentally responsible** hydrometallurgical techniques. These methods are designed to maximize recovery of target metals while minimizing impurities, reagent use, and waste generation.
--- ### **1. Advanced Leaching Methods**
#### **a) Pressure Acid Leaching (PAL)**
* **Use Case:** * Lateritic cobalt ores (e.g., in DRC, Indonesia).
* **How It Works:**
* Ore is mixed with concentrated sulfuric acid in an autoclave at high temperature (240–270 °C) and pressure (up to 4 MPa).
* **Benefits:** * High cobalt recovery (>95%).
* Ability to dissolve nickel and copper simultaneously for co-processing.
--- #### **b) Alkaline & Sulfate Roasting for Lithium**
* **Use Case:** * Hard rock spodumene ores.
* **How It Works:**
* Converts α-spodumene to β-spodumene via roasting at 1000–1100 °C, followed by leaching in Na₂SO₄ or Na₂CO₃ solution.
* **Benefits:**
* Lower acid consumption in downstream processing.
* Enables selective lithium extraction.
--- #### **c) Bioleaching**
* **Use Case:**
* Low-grade cobalt tailings and waste rock.
* **How It Works:**
* Bacteria such as *Acidithiobacillus ferrooxidans* generate acid and ferric ions to dissolve cobalt.
* **Benefits:**
* Low-energy, low-carbon footprint.
* Suitable for in-situ or heap leach applications.
--- #### **d) Direct Lithium Extraction (DLE) Leaching Variants**
* **Use Case:**
* Brines or leach liquors from clays and hard rock.
* **How It Works:** * Adsorbents, ion-exchange resins, or ceramic membranes selectively bind lithium ions from solution.
* **Benefits:**
* High selectivity → fewer impurities.
* Lower water use compared to evaporation ponds.
--- ### **2. Selective Extraction & Purification Techniques**
#### **a) Solvent Extraction (SX)**
* **For Cobalt:**
* Uses organophosphorus extractants (Cyanex 272, D2EHPA) to separate cobalt from nickel, copper, and manganese.
* **For Lithium:**
* Emerging SX systems use crown ethers or ionic liquids for high-purity lithium extraction.
#### **b) Ion Exchange (IX)**
* **Use Case:**
* Removal of trace contaminants (Mg²⁺, Ca²⁺) from lithium brines or concentrates.
* **Benefit:**
* Can be integrated with DLE for continuous polishing of product streams.
#### **c) Membrane Separation**
* **Types:**
* Nanofiltration (NF), Reverse Osmosis (RO), Electrodialysis (ED).
* **Application:**
* Pre-concentration of lithium brines, cobalt raffinate purification, water recycling.
--- ### **3. Advantages Over Conventional Methods**
* **Higher Selectivity:** Reduces co-dissolution of impurities.
* **Lower Reagent Consumption:** Optimized chemistry cuts acid/base use.
* **Better Environmental Performance:
** Smaller waste streams, reduced acid neutralization requirements.
* **Enables Co-Product Recovery:
** Nickel, copper, manganese can be recovered alongside lithium or cobalt.
Bioleaching of Lithium and Cobalt
--- ## **1. How It Works**
* Certain bacteria or fungi produce acids, chelating agents, or oxidizing agents that break down mineral lattices and release metals.
* For lithium and cobalt:
* **Lithium**: Often from lepidolite, hectorite, or recycled battery cathodes.
* **Cobalt**: Commonly from sulfide ores, mixed copper-cobalt ores, or battery waste.
--- ## **2. Microorganisms Used**
* **Acidophilic bacteria**:
*Acidithiobacillus ferrooxidans*, *Leptospirillum ferrooxidans* — oxidize sulfides to release cobalt.
* **Fungi**: *Aspergillus niger*, *Penicillium simplicissimum* — secrete organic acids (citric, oxalic) to dissolve lithium from silicates.
* **Alkaliphilic strains**: Under research for high-pH lithium recovery.
--- ## **3. Process Types**
1. **Direct Bioleaching**
– Microbes act directly on the ore surface, oxidizing and dissolving minerals.
2. **Indirect Bioleaching**
– Microbes generate acidic or oxidizing solutions that are then applied to the ore without the microbes being in direct contact.
--- ## **4. Advantages**
* Low energy consumption compared to roasting.
* Operates at mild temperatures (20–80 °C).
* Can process low-grade ores or tailings economically.
* Reduced chemical reagent use.
--- ## **5. Challenges**
* Slower leaching rates vs. chemical methods.
* Process control more complex (pH, temperature, microbial activity). * Some lithium silicates are highly resistant to biological attack.
--- ## **6. Current Applications & Research**
* **Cobalt bioleaching** is already used in the DRC for mixed Cu-Co sulfides.
* **Lithium bioleaching** mostly at pilot scale — particularly from spent Li-ion batteries, where organic acid-producing fungi have achieved >90% Li recovery.
* Hybrid **bio-hydrochemical circuits** are being tested: microbes liberate metals → solution polishing with solvent extraction or ion exchange.
Direct Lithium Extraction
These variants are being refined for higher efficiency, lower water use, and faster processing.
--- ## **1. Adsorption-Based Leaching**
* **Principle**: Uses lithium-selective sorbents (often manganese oxide or titanium-based) that adsorb lithium ions from solution, then release them during an elution stage.
* **Applications**:
* High-Mg/Li ratio brines (e.g., in Chile, Argentina, China). * Can treat geothermal brines or oilfield brines.
* **Advantages**: Low chemical use, selective for Li⁺ over Na⁺, Mg²⁺, Ca²⁺.
* **Challenge**: Sorbent fouling and regeneration cycle lifespan.
--- ## **2. Ion Exchange (IX) Leaching**
* **Principle**: Functionalized resins swap lithium ions with protons or sodium from the resin’s active sites.
* **Applications**: * Brines with high salinity.
* Post-bioleaching solutions from lithium-bearing clays.
* **Advantages**: Highly selective; modular and scalable.
* **Challenge**: Resin degradation from contaminants and scaling.
--- ## **3. Solvent Extraction (SX) Leaching**
* **Principle**: Organic extractants dissolve in an organic phase and selectively bind lithium from aqueous feed, followed by stripping into a clean aqueous phase.
* **Applications**:
* Brines with low lithium content but high impurity levels.
* Clay leach liquors.
* **Advantages**: High selectivity; produces concentrated Li solutions.
* **Challenge**: Handling organic solvents; emulsion formation.
--- ## **4. Membrane-Assisted Leaching**
* **Principle**: Uses nanofiltration (NF), reverse osmosis (RO), or lithium-ion–selective membranes to separate lithium from other salts.
* **Applications**:
* Pretreatment before adsorption or IX.
* Low-grade brines with high divalent ions.
* **Advantages**: Low chemical footprint; can operate continuously.
* **Challenge**: Membrane fouling, high upfront cost.
--- ## **5. Electrochemical DLE (Electrodialysis, Electrodeposition)**
* **Principle**: Lithium ions migrate through selective membranes under an applied electric field, concentrating lithium in one compartment.
* **Applications**:
* Geothermal brines.
* Post-leaching solutions from clays or tailings.
* **Advantages**: No chemical extractants; high purity lithium product.
* **Challenge**: Energy demand; membrane lifespan.
Selective extraction and purification Techniques
--- ## **1. Solvent Extraction (SX)**
* **Principle**: An organic extractant dissolved in a diluent selectively binds the target metal from an aqueous solution, followed by stripping into a clean aqueous phase.
* **Applications**:
* **Cobalt** recovery from copper raffinate in hydrometallurgical circuits.
* **Lithium** extraction from sulfate leach liquors.
* **Advantages**: High selectivity, scalable for large throughputs, well-established.
* **Challenges**: Organic solvent losses, emulsion control, disposal of spent organics.
--- ## **2. Ion Exchange (IX)**
* **Principle**: Functionalized resins exchange specific ions in solution with those on the resin’s active sites.
* **Applications**: * Removal of Mg²⁺, Ca²⁺, Na⁺ before lithium precipitation. * Selective cobalt recovery from nickel laterite leachates.
* **Advantages**: Highly selective, modular, low chemical use. * **Challenges**: Resin fouling, regeneration chemical costs.
--- ## **3. Precipitation Control**
* **Principle**: Adjusting pH, temperature, and reagent type to selectively precipitate the target metal or remove impurities first.
* **Applications**: * Cobalt hydroxide precipitation from SX raffinate.
* Lithium carbonate precipitation after brine concentration or DLE.
* **Advantages**: Simple, cost-effective, compatible with continuous operations.
* **Challenges**: Impurity co-precipitation, solid–liquid separation inefficiencies.
--- ## **4. Membrane Separation**
* **Principle**: Uses pressure-driven or electrochemical membranes to selectively pass target ions and reject others.
* **Applications**:
* Nanofiltration to remove divalent/trivalent impurities before lithium recovery.
* Electrodialysis for direct lithium separation from brines.
* **Advantages**: Low reagent footprint, continuous operation, high purity outputs.
* **Challenges**: Membrane fouling, capital cost.
--- ## **5. Hybrid Extraction Systems**
* **Principle**: Combines two or more methods—e.g., SX + IX, IX + membrane—to enhance selectivity and efficiency.
* **Applications**:
* Lithium–cobalt co-recovery circuits from complex ores.
* Cobalt recovery from tailings leachates with simultaneous impurity removal.
* **Advantages**: Maximizes recovery, reduces processing stages, adaptable to feed variability.
* **Challenges**: Higher process control requirements, integration complexity.
Solvent Extraction SX
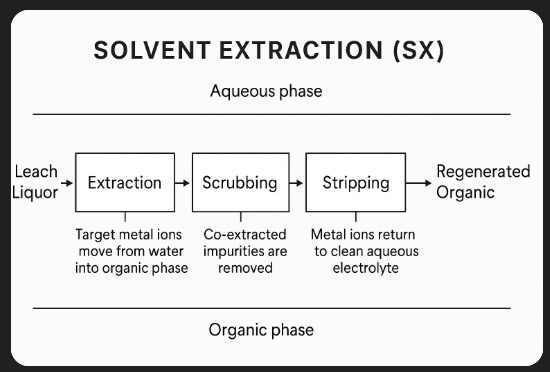
--- ## **1. Basic Principle**
* An **organic phase** (diluent + extractant) is brought into contact with an **aqueous phase** containing dissolved metals.
* The extractant selectively binds the target metal ions and transfers them into the organic phase.
* The loaded organic is then stripped with a new aqueous phase (often with different pH or chemical composition) to recover the metal in a purified form.
--- ## **2. Process Stages**
1. **Extraction** – Metal ions transfer from the aqueous leach liquor to the organic phase.
2. **Scrubbing** – Removes co-extracted impurities from the loaded organic using a weak aqueous wash.
3. **Stripping** – Target metal is transferred back to a fresh aqueous solution, producing a **high-purity electrolyte or solution** for further refining (e.g., electrowinning, precipitation).
4. **Regeneration** – Organic phase is reconditioned for reuse.
--- ## **3. Applications in Battery Metals**
* **Cobalt & Copper**: In DRC operations, SX is used to separate copper first, then extract cobalt from the raffinate.
* **Lithium**: SX can selectively extract lithium from high-sulfate or chloride brines after impurity removal.
* **Nickel–Cobalt Separation**: Specific extractants can separate nickel from cobalt in laterite or sulfide leach liquors.
--- ## **4. Advantages**
* High **selectivity** → enables recovery of metals from complex feeds.
* Produces **high-purity solutions** for refining.
* Continuous operation with good scalability.
--- ## **5. Challenges**
* Organic loss to aqueous phase (entrainment).
* Emulsion formation and phase separation issues.
* Spent extractant disposal or regeneration costs.
Ion Exchange IX
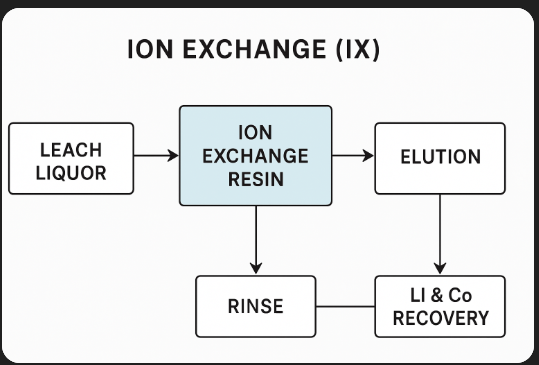
--- ### **How It Works**
1. **Feed Solution Contact**
– The leach liquor (containing dissolved metals) passes through a column or bed filled with ion-exchange resin beads.
2. **Adsorption** – Specific ions (e.g., Li⁺, Co²⁺, Ni²⁺) are selectively bound to the resin’s functional groups, replacing other ions originally on the resin.
3. **Rinse** – Impurities are washed away while the target ions remain bound to the resin.
4. **Elution (Desorption)** – A regenerant solution (often acid, base, or salt) displaces the target ions from the resin.
5. **Resin Regeneration** – The resin is restored to its original ionic form, ready for reuse.
--- ### **Applications in Lithium & Cobalt**
* **Lithium Brines & Geothermal Fluids** – IX resins selectively capture lithium even in the presence of high Mg/Ca content.
* **Cobalt Purification** – Removes impurities like manganese, iron, and copper before cobalt recovery.
* **Multi-Metal Recovery** – Different resin types can be staged to sequentially extract multiple valuable metals from the same feed.
--- ### **Advantages**
* High selectivity for target metals.
* Lower chemical consumption compared to precipitation.
* Operates at ambient temperature and pressure.
* Regenerable resins reduce waste.
Precipitation Control
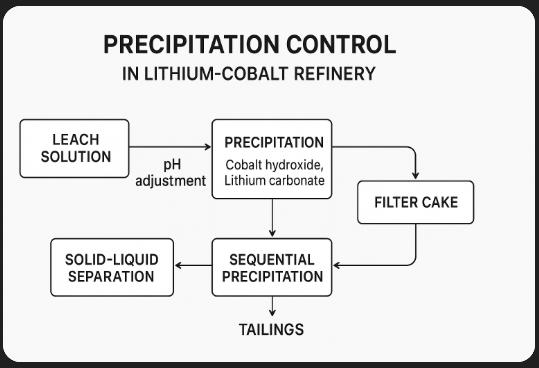
**Key aspects include:**
* **pH Adjustment:** Controlling acidity or alkalinity to trigger the precipitation of target compounds (e.g., cobalt hydroxide, lithium carbonate).
* **Reagent Choice & Dosage:** Using agents like lime, sodium carbonate, or sodium hydroxide at precise concentrations to promote selective precipitation.
* **Temperature & Mixing:** Higher temperatures can increase reaction rates, while good mixing ensures even reagent distribution.
* **Sequential Precipitation:** Removing one metal at a time by adjusting conditions in stages (e.g., iron first, then manganese, then cobalt).
* **Impurity Management:** Preventing unwanted co-precipitation that reduces purity or causes losses of the target metal.
* **Automation & Monitoring:** Using sensors and AI to dynamically adjust pH, ORP (oxidation-reduction potential), and reagent feed rates in real time.
Membrane separation
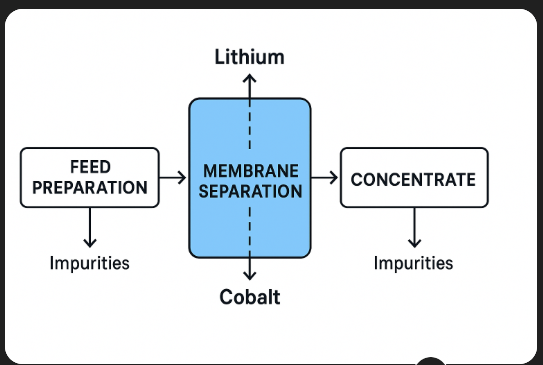
**Key Types:**
* **Nanofiltration (NF):** Removes divalent/trivalent impurities while allowing monovalent lithium ions to pass.
* **Reverse Osmosis (RO):** High-pressure process for removing almost all dissolved salts and impurities.
* **Electrodialysis (ED):** Uses ion-exchange membranes and electric potential to separate charged lithium and cobalt species.
* **Membrane Electrodeionization (MEDI):** Combines membranes with resin for high-purity product polishing.
**Advantages:**
* Low chemical consumption compared to precipitation.
* Compact footprint.
* High selectivity for target ions.
**Challenges:**
* Membrane fouling from suspended solids or scaling salts.
* Limited tolerance for extreme pH or organic solvents.
* Requires pre-treatment to maintain performance.